Methotrexate: patient drug information
Methotrexate: Patient drug information Obtained from Uptodate. U.S. Brand Names: Rheumatrex®; Trexall™ Mexican Brand Names: Atrexel; Ledertrexate; Medsatrexate; Otaxem; Texate; Trixilem Pharmacologic Category: Antineoplastic Agent, Antimetabolite (Antifolate); Antirheumatic, Disease Modifying What key warnings should I know about before taking this medicine? • Your bone ma
 Molecular Membrane Biology, September Á/October 2004, 21, 307 Á/313
pH modulation of large conductance potassium channel from adrenalchromaffin granules
channel gene CLCN7 leads to a severe osteopetrotic
phenotype because osteoclasts fail to resorb bone and
they cannot acidify the lacuna [6]. Mitochondrial potassiumchannel has been suggested as a trigger and effectormyocardial ischemic preconditioning [7].
Molecular Membrane Biology, September Á/October 2004, 21, 307 Á/313
pH modulation of large conductance potassium channel from adrenalchromaffin granules
channel gene CLCN7 leads to a severe osteopetrotic
phenotype because osteoclasts fail to resorb bone and
they cannot acidify the lacuna [6]. Mitochondrial potassiumchannel has been suggested as a trigger and effectormyocardial ischemic preconditioning [7].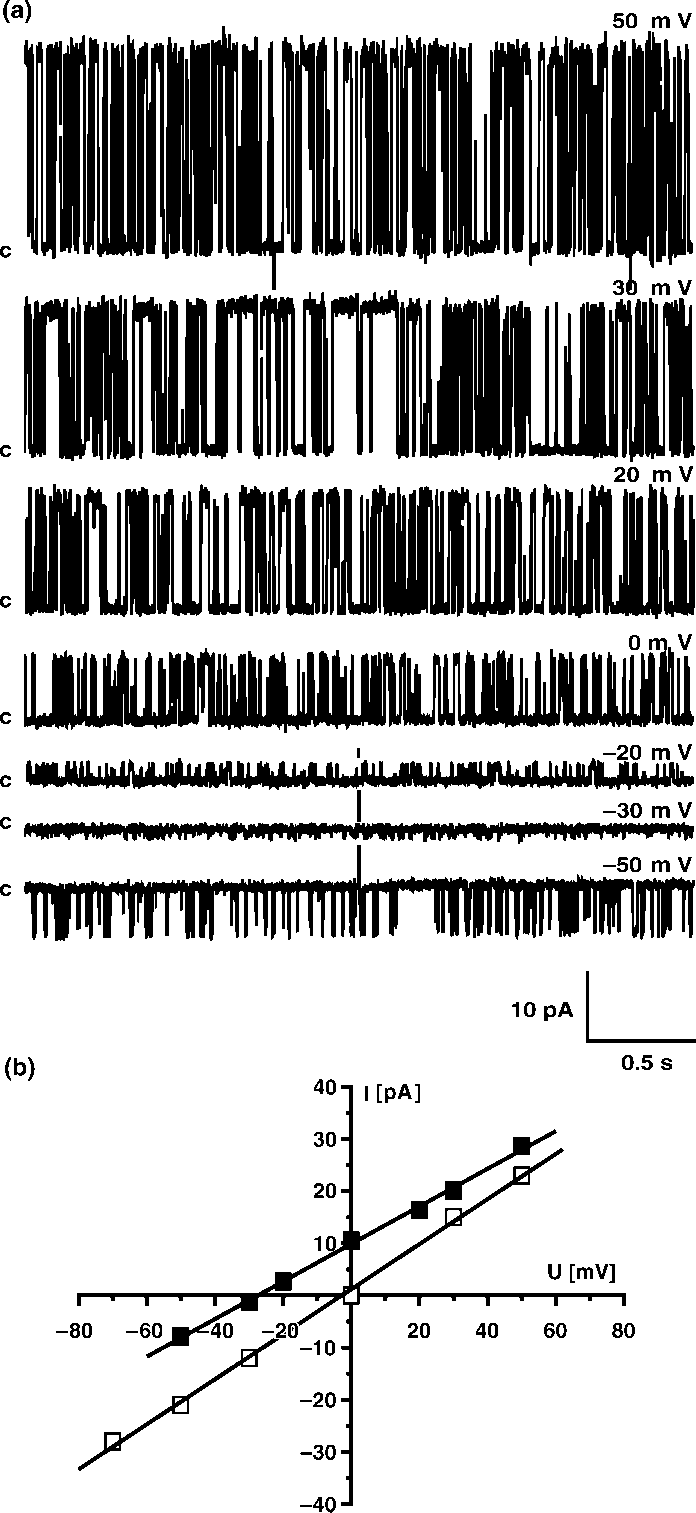
 Calculated probability of opening for 50 mV in symmetrical450 mM KCl was Popen0/0.519/0.15 whereas for (/50 mVwas only Popen0/0.119/0.07. Such a result clearly indicatedthat potassium channel with large conductance is voltagedependent. We also performed gating analysis of singlechannel recording at 50 mV and (/50 mV in symmetrical450 mM KCl, pH 7.0. Single current amplitudes at 9/50 mVobtained from histograms had the same values ( Â/229/1 pA)whereas strong differences were observed in the closed- andopen-time distributions. The calculated open-times for thelarge conductance potassium channel investigated are:to 0/9.749/0.37 ms at 50 mV but only to 0/3.859/0.31 ms at
(/50 mV. The effect was more evident for the closed-times:
tc0/10.189/0.38 ms at 50 mV and tc0/31.989/0.92 ms at
(/50 mV, suggesting that the channel is voltage dependent.
Calculated probability of opening for 50 mV in symmetrical450 mM KCl was Popen0/0.519/0.15 whereas for (/50 mVwas only Popen0/0.119/0.07. Such a result clearly indicatedthat potassium channel with large conductance is voltagedependent. We also performed gating analysis of singlechannel recording at 50 mV and (/50 mV in symmetrical450 mM KCl, pH 7.0. Single current amplitudes at 9/50 mVobtained from histograms had the same values ( Â/229/1 pA)whereas strong differences were observed in the closed- andopen-time distributions. The calculated open-times for thelarge conductance potassium channel investigated are:to 0/9.749/0.37 ms at 50 mV but only to 0/3.859/0.31 ms at
(/50 mV. The effect was more evident for the closed-times:
tc0/10.189/0.38 ms at 50 mV and tc0/31.989/0.92 ms at
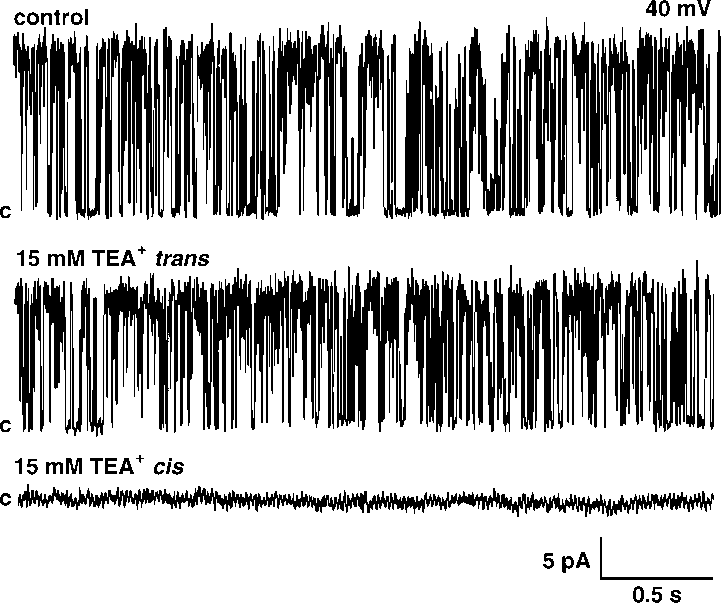
(/50 mV, suggesting that the channel is voltage dependent.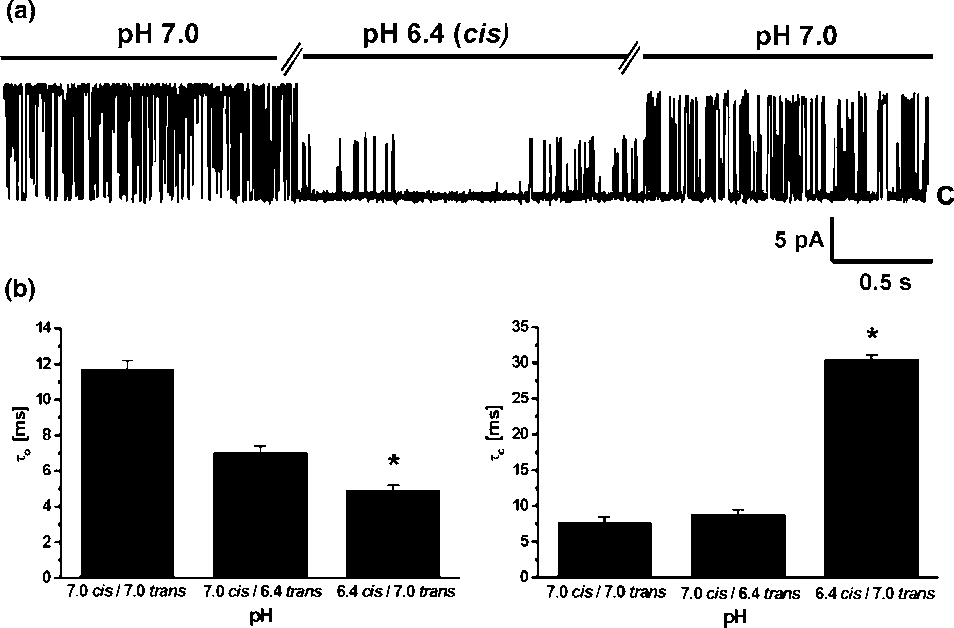 placed into the trans -bilayer chamber results in current
increased to tc0/8.89/0.3 ms but after lowering it in the cis
amplitude reduction at 20% without changing open prob-
side it increased to tc0/30.59/2.9 ms.
placed into the trans -bilayer chamber results in current
increased to tc0/8.89/0.3 ms but after lowering it in the cis
amplitude reduction at 20% without changing open prob-
side it increased to tc0/30.59/2.9 ms.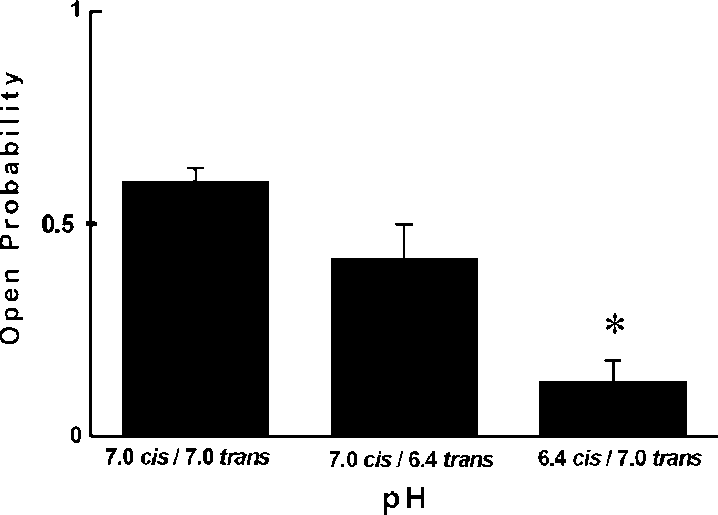 both insensitive to ChTX and Ca2'. Our results indicate thatthe channel activity observed in the present paper wassimilar to one described by Ashley et al . (1994). Similar to86Rb' flux experiments we observed a strong inhibition ofthe K' channel by low pH. Interestingly, this strong effectwas observed only from the cis side. The inhibition of the K'channel by lowering pH was observed from the same side asTEA' inhibition. Previously it was shown that a chromaffingranule K' channel is blocked by TEA' from the intragra-nular side [12]. This suggests that the effect of pH is alsofrom the intragranular side. The findings of the present studymay be important for our understanding of the physiologicalrole of potassium conductance in chromaffin granules.
both insensitive to ChTX and Ca2'. Our results indicate thatthe channel activity observed in the present paper wassimilar to one described by Ashley et al . (1994). Similar to86Rb' flux experiments we observed a strong inhibition ofthe K' channel by low pH. Interestingly, this strong effectwas observed only from the cis side. The inhibition of the K'channel by lowering pH was observed from the same side asTEA' inhibition. Previously it was shown that a chromaffingranule K' channel is blocked by TEA' from the intragra-nular side [12]. This suggests that the effect of pH is alsofrom the intragranular side. The findings of the present studymay be important for our understanding of the physiologicalrole of potassium conductance in chromaffin granules.