Wipo arbitration and mediation center
Before the: WORLD INTELLECTUAL PROPERTY ORGANIZATION ARBITRATION AND MEDIATION CENTER LATIN AMERICAN TELECOM, LLC (Objector) TLD string objected to: < .TUBE > (Applicant/Respondent) LEGAL RIGHTS OBJECTION (Applicant Guidebook, Module 3; Procedure, art. 6, 7, 8; WIPO Rules for New gTLD Dispute Resolution, para. 4) I. Introduction [1.] This Legal
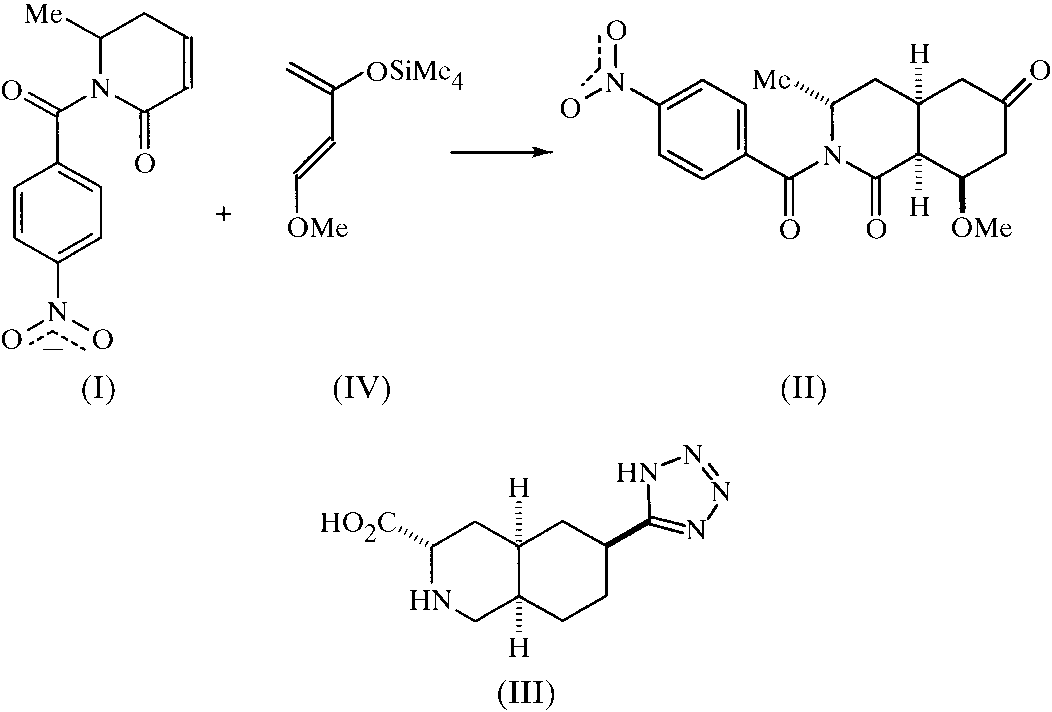
 mate, the excitatory amino acid antagonist activities of a series
of decahydroisoquinoline-3-carboxylic acids were explored. It
was found that compound (III) possesses both NMDA and
AMPA receptor antagonist activity (Simmons et al., 1998;
A new route to the synthesis of (III) was proposed, based
on an intermolecular Diels±Alder cycloaddition reaction of a
6-substituted dihydropyridone with an appropriate diene. This
and AMPA receptor antagonist activity key reaction would need a group at position 6 (to the nitro-
gen) in an axial position, so before the cycloaddition reaction
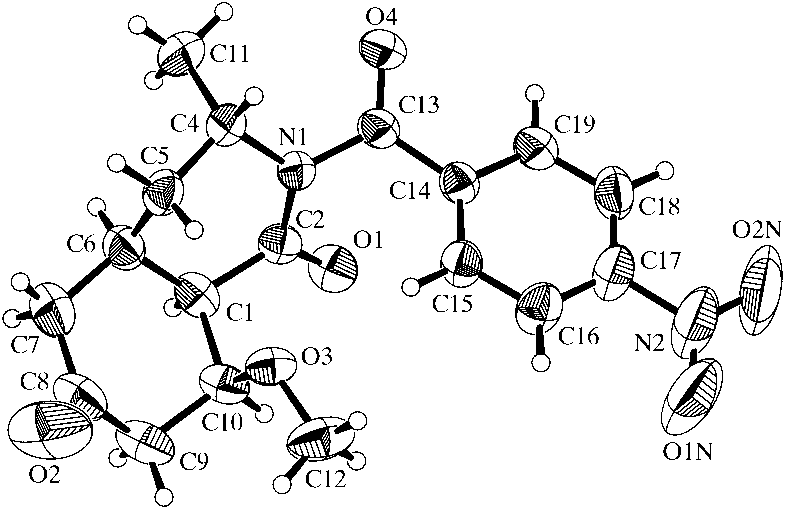
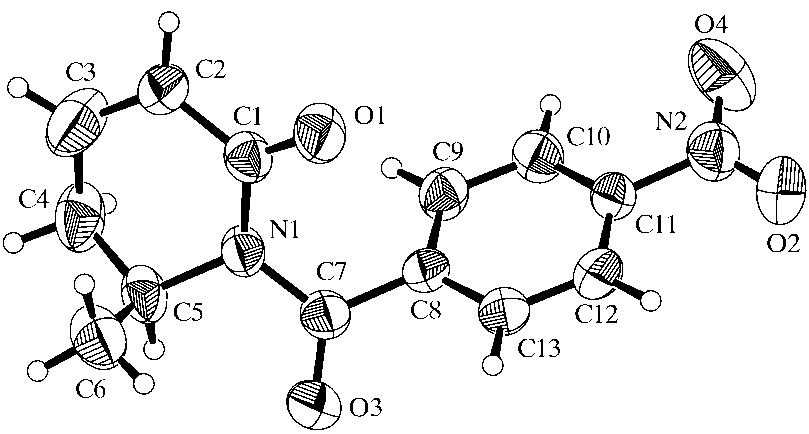
was attempted, a crystal structure determination of (I) was
J. Zukerman-Schpector,a,b* Mauricio Vega,b I. Caracelli,b
undertaken. After con®rmation that the methyl group (at
Luiz C. Diasc and Anna M. A. P. Fernandesc
position 6, labelled C5 in the ®gure) occupied an axial posi-
tion, a thermally induced Diels±Alder reaction was performed
aInstituto de QuõÂmica, Universidade de SaÄo Paulo, SaÄo Paulo, SP, Brazil,
using the highly reactive Danishefsky's diene, (IV). As the
bLaboratoÂrio de Cristalografia, EstereodinaÃmica e Modelagem Molecular,
Diels±Alder reaction could lead to several different products
Departamento QuõÂmica, Universidade Federal de SaÄo Carlos, Caixa Postal 676,13565-905 SaÄo Carlos, SP, Brazil, and cInstituto de QuõÂmica, UNICAMP, Caixa
and as the relative stereochemistry of this product is of great
Postal 6154, 13083-970 Campinas, SP, Brazil
importance for subsequent reaction steps, the crystal structure
Correspondence e-mail:
mate, the excitatory amino acid antagonist activities of a series
of decahydroisoquinoline-3-carboxylic acids were explored. It
was found that compound (III) possesses both NMDA and
AMPA receptor antagonist activity (Simmons et al., 1998;
A new route to the synthesis of (III) was proposed, based
on an intermolecular Diels±Alder cycloaddition reaction of a
6-substituted dihydropyridone with an appropriate diene. This
and AMPA receptor antagonist activity key reaction would need a group at position 6 (to the nitro-
gen) in an axial position, so before the cycloaddition reaction
was attempted, a crystal structure determination of (I) was
J. Zukerman-Schpector,a,b* Mauricio Vega,b I. Caracelli,b
undertaken. After con®rmation that the methyl group (at
Luiz C. Diasc and Anna M. A. P. Fernandesc
position 6, labelled C5 in the ®gure) occupied an axial posi-
tion, a thermally induced Diels±Alder reaction was performed
aInstituto de QuõÂmica, Universidade de SaÄo Paulo, SaÄo Paulo, SP, Brazil,
using the highly reactive Danishefsky's diene, (IV). As the
bLaboratoÂrio de Cristalografia, EstereodinaÃmica e Modelagem Molecular,
Diels±Alder reaction could lead to several different products
Departamento QuõÂmica, Universidade Federal de SaÄo Carlos, Caixa Postal 676,13565-905 SaÄo Carlos, SP, Brazil, and cInstituto de QuõÂmica, UNICAMP, Caixa
and as the relative stereochemistry of this product is of great
Postal 6154, 13083-970 Campinas, SP, Brazil
importance for subsequent reaction steps, the crystal structure
Correspondence e-mail: