math.utoledo.edu
Department of MathematicsThe University of Toledo2801 W. Bancroft St Toledo, Ohio 43606-3390Tel:(O)(419) 530-2998 Fax: (419) 530-4720Email:[email protected]:http://www.math.utoledo.edu/∼mtsuiDifferential Geometry (53), General Relativity and Gravitation (83) andPartial Differential Equations (35). • Ph. D. in Mathematics, 2001, Brandeis University• M.S. in Applied Mathemat
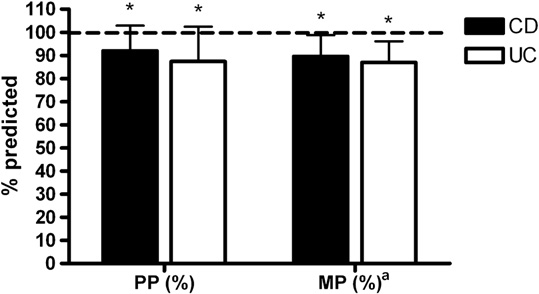
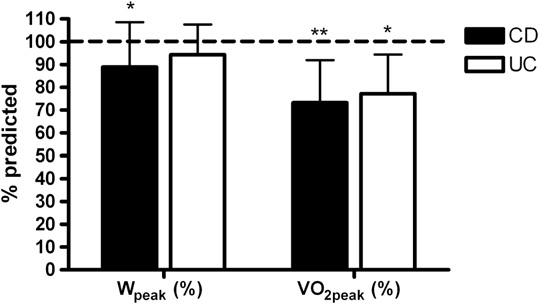 ter controlling for age, hemoglobin remained significantlycorrelated with PP (Watts$kg-1; r = 0.45, P = .049), MP(Watts$kgÀ1; r = 0.63, P = .003), Wpeak (Watts$kgÀ1; r =.70, P = .001), VO2peak (L$min–1; r = 0.60, P = .006), andVO2peak (mL$kgÀ1$min–1; r = 0.62, P = .004).
ter controlling for age, hemoglobin remained significantlycorrelated with PP (Watts$kg-1; r = 0.45, P = .049), MP(Watts$kgÀ1; r = 0.63, P = .003), Wpeak (Watts$kgÀ1; r =.70, P = .001), VO2peak (L$min–1; r = 0.60, P = .006), andVO2peak (mL$kgÀ1$min–1; r = 0.62, P = .004).